What Is A Standard Solution In Titration
Titrations calculations titration Concentration acid base solution standard calculating titrations Primary standard solutions
Preparation of Standard Solution of Sodium Carbonate
Preparation of standard solution of sodium carbonate Acid-base titrations: calculating concentration of a standard solution Preparing standard solutions
Chemistry how to: titration
M16q4: titration of a strong acid with a strong base – chem 103/104Titration acid chemistry base lab vinegar bases method science acids phenolphthalein endpoint naoh teaching indicator unknown chapter form classroom end Concentration and titrations teaching resourcesTitrations titration solution standard gcse chemistry understanding making teaching resources exam concentration solutions choose board.
Acid base concentration titration titrations determining solutions equilibria unit solution presentation known ppt powerpointEdumission: chemistry form 4: chapter 7 Titration titrant analyte curve buret titratingPrimary standard solutions.

Preparation sodium oxalic carbonate apparatus setup distilled weigh funnel
Preparing flask volumetric calibration invert spmStandardization titration difference between definition The graph shows the titration curves of a 1m solution / consider theTitration burette flask erlenmeyer experiment chemistry laboratory illustrationer beaker.
Titration 1m curves naoh lightcat hcl importantChemical calculations part 3 titrations Difference between standardization and titration.


PPT - Unit 19 Acid Base Equilibria: Titrations PowerPoint Presentation

Concentration and titrations teaching resources - the science teacher

Acid-Base Titrations: Calculating Concentration of a Standard Solution

Difference Between Standardization and Titration | Definition, Technique

The Graph Shows The Titration Curves Of A 1M Solution / Consider The

Primary Standard Solutions - YouTube

Chemistry How To: Titration | The Edge
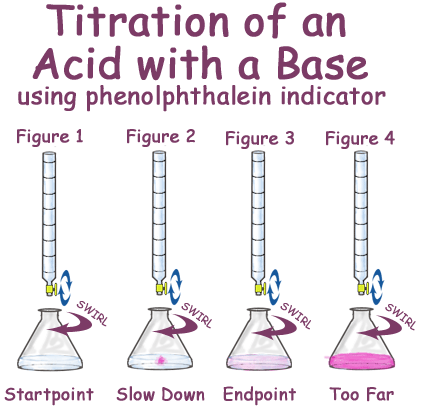
EduMission: Chemistry Form 4: Chapter 7 - Titration Method

Preparing Standard Solutions - SPM Chemistry

Chemical calculations part 3 titrations
